
Abstract
Mesenchymal stem cell (MSC) therapy is a frequently used treatment option for achieving a better prognosis in patients with heart failure (HF). However, due to reported adverse effects, patients are often hesitant to consider this treatment. Consequently, the aim of this systemic review and meta-analysis is to further investigate the effects of MSCs on survival outcomes, hospital readmissions, and left ventricular ejection fraction (LVEF) in individuals with pre-existing HF. We systematically searched PubMed, Web of Science, Embase, and Cochrane Library to review studies published up until July 16, 2023. Risk ratios were generated using the extracted data for all the outcomes except LVEF. The mean difference was generated for LVEF. Sensitivity analysis was performed to investigate heterogeneity, and the risk of bias tool was used to assess the quality of the included studies. Fourteen randomized controlled trials were included in the meta-analysis. Pooled results revealed that the MSC therapy group did not significantly affect the outcomes of cardiovascular death, rehospitalization rate, myocardial infarction, recurrence of HF, and total death when compared to a control group. However, MSC therapy was significantly associated with an increased LVEF (RR = 3.35; 95% CI: 0.79-5.72; p = 0.010; I2 = 95%). Upon sensitivity analysis, MSC therapy was significantly associated with a decreased hospitalization rate (RR = 0.46; 95% CI: 0.34-0.64; p < 0.00001; I2 = 0%). MSC transplantation results in a significantly improved LVEF and rehospitalization rate.
Introduction & Background
Heart failure (HF) is a pathological medical condition that occurs due to the heart failing to pump an adequate amount of blood for the body. This results from either decreased ventricular ejection or the inability of the ventricle to accommodate normal venous return [1]. It has been approximated that HF is the cause of 266,400 deaths annually, and the incidence of HF may increase by 46% (from 2012) until 2030 [2,3]. Furthermore, HF can worsen lifestyle by impairing kidney function and liver function and causing pulmonary hypertension, pulmonary edema, or cardiac arrhythmia [4]. It is thus crucial to focus attention on treatment methods for patients with HF to achieve reduced mortality and control worsening organ function in these individuals.
One such treatment option is the use of mesenchymal stem cells (MSCs). MSCs have been used for many years to improve the prognosis in HF patients [5]. MSCs are a type of stromal cells that can undergo mitosis to replace other degenerated MSCs and can differentiate into a wide variety of other cells. They can thus be easily found in abundance in the bone marrow, adipose tissue, lung tissue, synovial membrane, endometrium, and blood [6].
It has been proposed that the therapeutic effect of MSCs in patients with HF and other cardiovascular diseases may be due to their capability to differentiate into cardiovascular cells, their ability to stimulate the immune system, and their antifibrotic and angiogenetic properties [7]. Through these mechanisms, MSCs have been correlated with a significant improvement in left ventricular ejection fraction (LVEF). LVEF is often used to assess the degree and type of HF (systolic or diastolic). An LVEF of less than 45% is an excellent predictor of increased mortality in patients with cardiovascular disease [8]. Thus, an increase in LVEF with MSCs indicates improved heart function and better survival outcomes in HF patients.
However, due to the emergence of adverse effects with MSCs use, some individuals are reluctant to use them, and thus ongoing research is being conducted regarding their administration. Some studies suggest that MSCs administration can lead to fever, fatigue, sleeplessness, diarrhea, dermatitis, or vascular disorders [9]. Moreover, while existing literature attempts to investigate the association between MSCs and survival outcomes in HF patients, the reported findings are inconsistent.
Some studies suggest that the administration of MSCs in HF patients is safe and advisable, yielding a better prognosis [10-17]. However, other studies indicate no significant difference in survival outcomes or LVEF in HF patients undergoing stem cell therapy [18,19]. Consequently, we conducted a systematic review and meta-analysis to assess the effect of MSC therapy on outcomes among HF patients.
Review
Methods
This systematic review and meta-analysis adhered to the guidelines outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [20].
Search Strategy
Two authors conducted independent searches through electronic databases, including PubMed, Web of Science, Embase, and Cochrane Library, to review studies published up until July 16, 2023. Additionally, previous meta-analyses were also reviewed, and relevant studies were extracted. There were no restrictions placed on the geographical area, year of publication, or publication type during the literature review process. The following key terms and words analogous to them were used to search existing literature and identify relevant articles: "mesenchymal stem cell therapy," "mesenchymal stem cells," and "heart failure," along with the Boolean operators "AND" and "OR." Any disagreement regarding the study selection was resolved by consultation with a third author. For further details regarding the search strategy and study selection process, refer to Figure 1.
PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
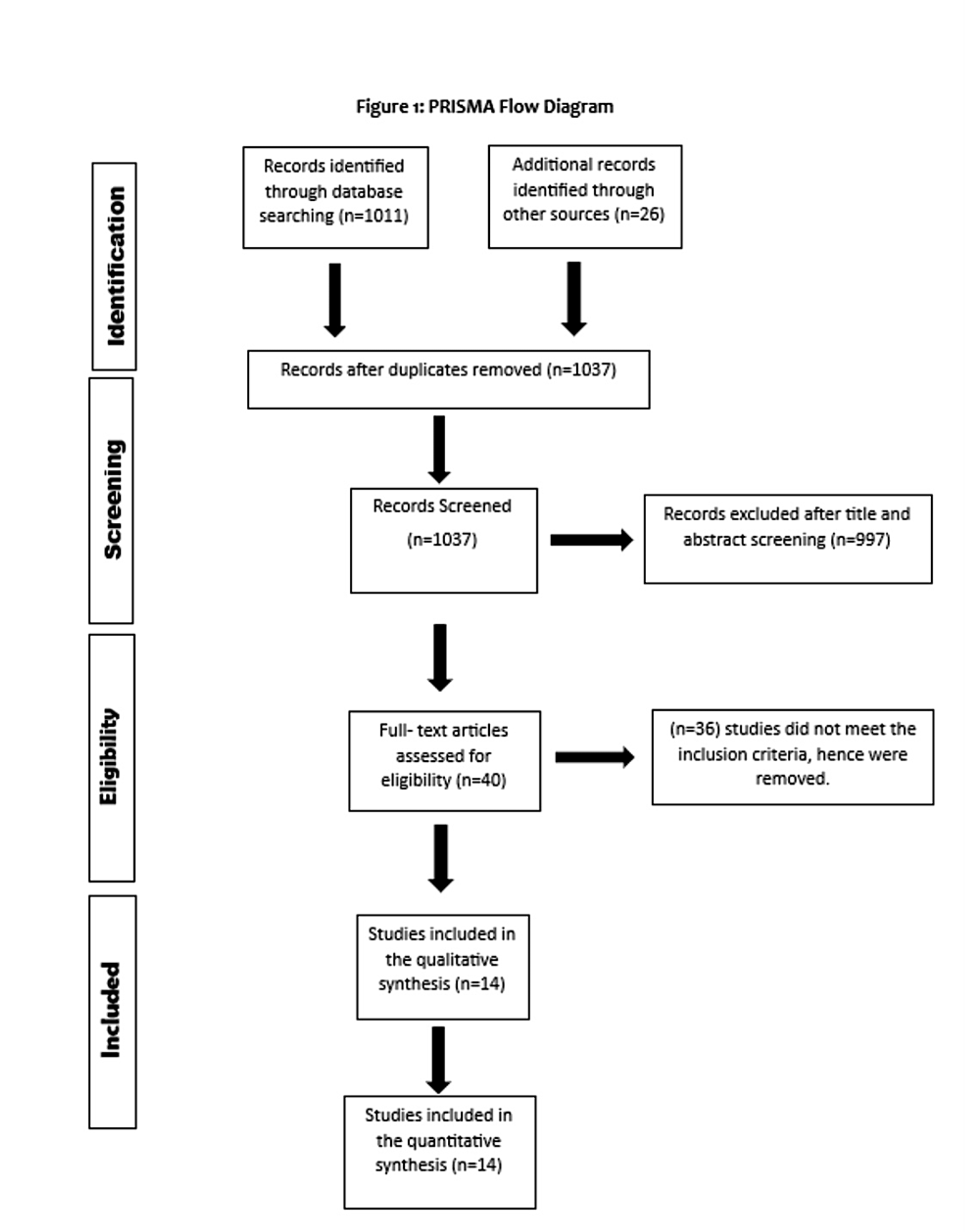
Figure 1: PRISMA flowchart of study selection
Study Selection
Studies were included in this meta-analysis based on the following eligibility criteria: (1) studies that were randomized controlled trials (RCTs); (2) studies that examined the effect of MSCs in HF patients; (3) studies that included a control group. All of the included studies were compiled and checked to remove any existing duplicates.
Data Extraction and Quality Assessment
Two reviewers independently extracted relevant data. The following data were extracted: the name of the first author, the year of publishing, publication type, population size, type of MSCs, the method of MSCs administration, mean age of participants, number of males, BMI, New York Heart Association (NYHA) class, and follow-up time. The events/total for all outcomes were also extracted. Our primary outcome was LVEF, while our secondary outcomes were the incidence of cardiovascular death, rehospitalizations, MI, recurrence of HF, and total death. We assessed the quality of all included RCTs using the risk of bias tool [21]. A summary of the results of our quality assessment is available in Appendix A.
Statistical Analysis
Statistical analyses were performed using Review Manager software, version 5.3 (Cochrane Collaboration, Copenhagen, Denmark). The association between MSC therapy and adverse or beneficial outcomes in HF patients was evaluated by collecting relevant data and calculating the corresponding mean difference or risk ratio with a 95% confidence interval (CI) for all outcomes. The results of these analyses were presented in forest plots using a random-effects model. Study heterogeneity was assessed using the I2 statistic. A p-value less than 0.05 was considered statistically significant. Sensitivity analysis was also conducted to address the heterogeneity in the results.
Results
Figure 1 shows the PRISMA flowchart of study selection. Initially, 1,037 possibly pertinent articles in total were found. Duplicates were removed. Finally, the meta-analysis included 14 RCTs that satisfied our inclusion criteria.
The baseline characteristics of the included studies are listed in Table 1. The total number of patients was 1,445, including 83.6% males with a mean age of 41.9 years. The methods for the application of MSC were intracoronary transplantation, intramyocardial injection, and intravenous infusion. The follow-up time for all the included RCTs was more than six months [22-38].
Author, year | Study type | Number of participants (MSC group/control group) | Mean age of participants (MSC group/control group) | Number of males (MSC group/control group) | BMI (MSC group/control group) | NYHA class III and IV (MSC group/control group) | Method of stem cell delivery | Type of MSC | Type of HF | Patient population | Control group | Follow-up time |
Ascheim et al. (2014) [10] | Multicenter, double-blind, sham-procedure controlled trial | 20/10 | 55.1 ± 15.4/62.2 ± 7.8 | 17/8 | NA | 3 and 17/2 and 7 | Intramyocardial injection of allogeneic MPCs | Allogeneic MPCs, adult bone marrow-derived mononuclear cells | End-stage heart failure, of either ischemic or nonischemic etiology | Recipients of contemporary left ventricular assist devices (adults with end-stage heart failure) | Cryoprotective medium | Until transplant or 12 months after randomization |
Bartolucci et al. (2017) [11] | A phase 1/2 randomized controlled trial | 15/15 | 57.33 ± 10.05/57.20 ± 11.64 | 12/14 | 29.12 ± 2.88/29.52 ± 4.00 | NA | Intravenous infusion of UC-MSCs | Umbilical cord mesenchymal stem cells | Chronic HFrEF | Patients with stable heart failure and reduced ejection fraction | Placebo | 3, 6, and 12 months post-therapy |
Bartunek et al. (2013) [12] | Prospective, multicenter, randomized trial | 21/24 | 55.7 ± 10.4/59.5 ± 8.0 | 20/22 | NA | NA | Endomyocardial injection of autologous bone marrow-derived and cardiogenically oriented mesenchymal stem cells | Autologous bone marrow-derived and cardiogenically oriented mesenchymal stem cell | Heart failure of ischemic origin | Heart failure of ischemic origin | Beta-blocker, an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker, and a diuretic | 6 months post-therapy, 2 years post-therapy |
Bartunek et al. (2017) [18] | Multinational, randomized, double-blind, sham-controlled study | 120/151 | 61.6 ± 8.6/62.1 ± 8.7 | 107/136 | 28.2 ± 3.7/28.6 ± 4.4 | 96 and 1/114 and 1 | Cardiopoietic cells delivered endomyocardially with a retention-enhanced catheter | Bone marrow mesenchymal stem cells | Heart failure of ischemic origin | Patients with symptomatic ischemic heart failure | Insertion of an introducer sheath, left ventricular angiography, and pigtail catheter movements | 26 and 39 weeks |
Bolli et al. (2021) [13] | Double-blind, placebo-controlled, phase II trial | 29/32 | 61.7 ± 6.7/63.1 ± 8.8 | 27/31 | 30.4 ± 5.4/30.0 ± 4.4 | 6/3 | Transendocardial injection of MSCs | Autologous bone marrow-derived mesenchymal stromal cells | Heart failure of ischemic origin | Patients with ischemic heart failure | Placebo | 12 months |
Butler et al. (2017) [36] | Single-blind, placebo-controlled, crossover, randomized phase II-a trial | 22 (combined group) | 47.3 ± 12.8 (combined group) | 13 (combined group) | 32.24 ± 7.56 (combined group) | 1 (combined group) | Intravenously administered ischemia-tolerant MSCs | Ischemia-tolerant MSCs | Heart failure of non-ischemic origin | Patients with nonischemic cardiomyopathy | Placebo | 90 days |
Heldman et al. (2014) [14] | Phase 1 and 2 randomized, blinded, placebo-controlled study | 19/11 | 57.1 ± 10.6/60.0 ± 12.0 | 18/10 | NA | 2/3 | Transendocardial injection of autologous mesenchymal stem cells | Autologous mesenchymal stem cells (MSCs) and bone marrow mononuclear cells | Heart failure of ischemic origin | Patients with ischemic cardiomyopathy and left ventricular (LV) ejection fraction of less than 50% | Placebo | 30 days, 1-year post-therapy |
Kim et al. (2018) [37] | RCT | 14/12 | 55.3 ± 8.6/57.8 ± 8.9 | 14/12 | NA | NA | Intracoronary delivery of autologous bone marrow mesenchymal stem cells | Autologous bone marrow-derived mesenchymal stromal cells | Congestive HF | Patients with anterior wall ST-segment elevation myocardial infarction | Optimum post-infarction treatment | 4 months |
Mathiasen et al. (2015) [15] | Randomized, double-blind, placebo-controlled trial | 40/20 | 66.1 ± 7.7/64.2 ± 10.6 | 36/14 | 29.8 ± 4.7/28.7 ± 5.3 | 29/15 | Intra-myocardial injections | Autologous bone marrow-derived MSCs | Heart failure of ischemic origin | Patients with severe ischemic heart failure | Placebo | 1 month, 3 months, 6 months |
Perin et al. (2015) [17] | Phase 2, multicenter, dose-escalation study | 45/15 | 62.2 ± 10.3/62.7 ± 11.2 | 44/11 | 29.8 (4.1)/31.3 (9.2) | 14 and 0/9 and 0 | Transendocardial injection of allogeneic MPCs | Allogeneic MPCs, adult bone marrow-derived mononuclear cells | Heart failure due to left ventricular systolic dysfunction of either ischemic or nonischemic etiology | Patients with chronic heart failure | Mock mapping/injection procedures | 13 months post-therapy, 3 years post-therapy |
Perin et al. (2023) [16] | Randomized, double-blind, multicenter study | 283/282 | 62.7 ± 10.9/62.6 ± 10.4 | 222/221 | NA | 175/178 | Transendocardial injection of allogeneic MPCs | Allogeneic MPCs, adult bone marrow-derived mononuclear cells | Heart failure (ischemic or nonischemic) | Heart failure with reduced ejection fraction (HFrEF) | Patients who did not receive stem cell therapy or any placebo transendocardial injections | 12 months post-therapy |
Xiao et al. (2017) [38] | Randomized comparative study | 17/20 | 51.6 ± 12.2/54.4 ± 11.6 | 12/14 | NA | NA | Intracoronary injection | Bone marrow mesenchymal stem cells | Diastolic HF | Patients with dilated cardiomyopathy | Saline | 3 months, 12 months |
Yau et al. (2019) [19] | Randomized phase 2 clinical trial | 106/53 | 55.5 ± 12.3/56.9 ± 11.7 | 94/47 | NA | 31 and 75/12 and 41 | Intramyocardial injection of allogeneic MPCs | Allogeneic MPCs, adult bone marrow-derived mononuclear cells | End-stage heart failure (ischemic or nonischemic) | Recipients of contemporary left ventricular assist devices (adults with end-stage heart failure) | Cryoprotective medium | 6 months post-therapy, 1 year post-therapy |
Zhao et al. (2015) [35] | RCT | 30/29 | 52.90 ± 16.32/53.2 ± 11.46 | 24/19 | NA | NA | Intracoronary injection of umbilical cord mesenchymal stem cells | Umbilical cord mesenchymal stem cells | Chronic systolic heart failure | Patients with severe systolic HF | Medication | 1 and 6 months post-therapy |
Table 1: Baseline characteristics of included studies
MSC: mesenchymal stem cell; MPCs: mesenchymal precursor cell; UC-MSCs: umbilical cord-derived mesenchymal stem cells; RCT: randomized controlled trial; HF: heart failure; HFrEF: heart failure with reduced ejection fraction; LV: left ventricle.
Primary Outcome: Cardiovascular Death
The random-effects model was used to analyze the primary outcome data. The six included RCTs' pooled estimates indicated that the MSC intervention did not significantly affect cardiovascular death when compared to the control group for HF (RR = 0.85; 95% CI: 0.61-1.19; p = 0.34) (Figure 2). The heterogeneity between the studies was also low (I2 = 0%; heterogeneity p = 0.48).
Figure 2: Forest plot for the meta-analysis of cardiovascular death
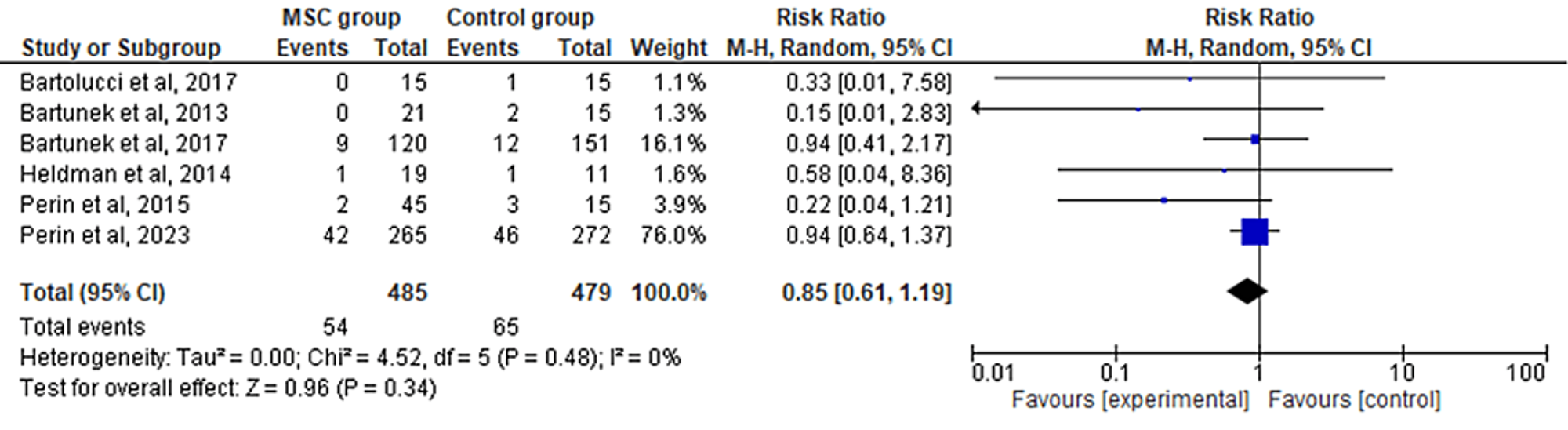
Favors experimental: mesenchymal stem cell (MSC) group.
Secondary Outcomes: LVEF
The 11 included RCTs' pooled estimates indicated that the MSC intervention was associated with a significantly increased LVEF when compared to the control group (RR = 3.35; 95% CI: 0.79-5.72; p = 0.010; I2 = 95%) (Figure 3). To address the heterogeneity in the results, sensitivity analysis was conducted. The results remained consistent, but the heterogeneity lowered considerably (I2 = 0%; heterogeneity p = 0.48) (Appendix B).
Figure 3: Forest plot for the meta-analysis of left ventricular ejection fraction (%)
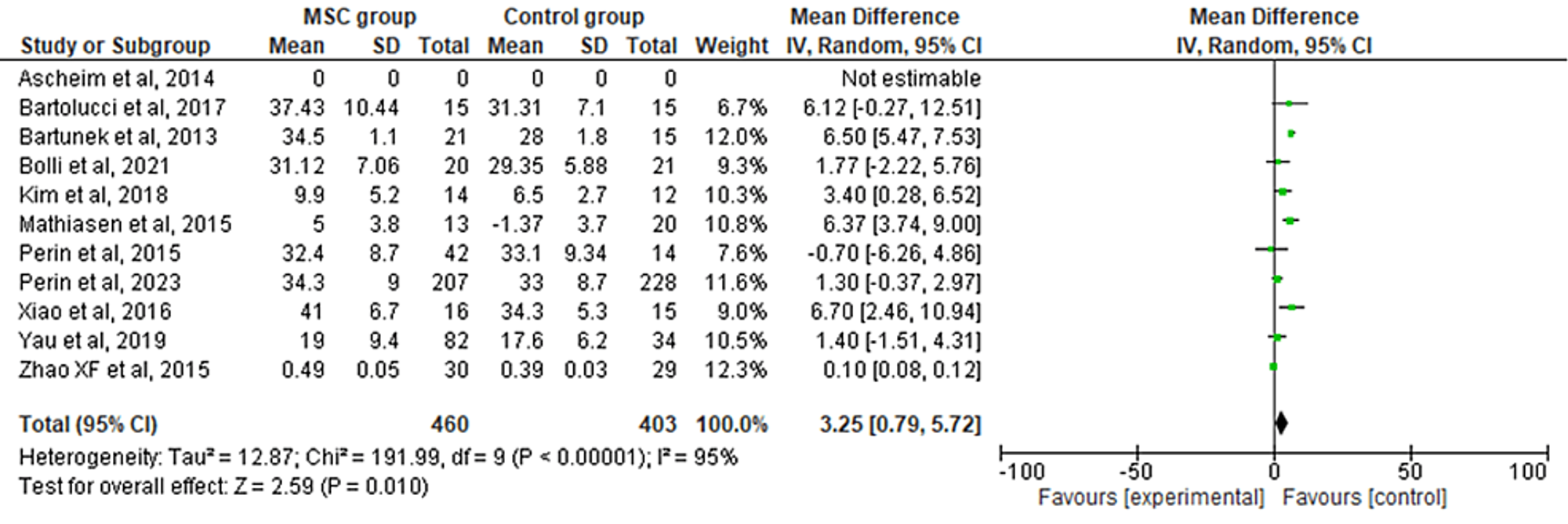
Favors experimental: mesenchymal stem cell (MSC) group.
Rehospitalization Rate
The 10 included RCTs' pooled estimates indicated that there was no significant difference between the MSC intervention group versus the control group for the outcome of rehospitalization rate (RR = 0.55; 95% CI: 0.29-1.06; p = 0.07; I2 = 87%) (Figure 4). Upon conducting sensitivity analysis, the results differed, favoring the MSC therapy group over the control group while the heterogeneity also decreased (RR = 0.46; 95% CI: 0.34-0.64; p < 0.00001; I2 = 0%) (Appendix C).
Figure 4: Forest plot for the meta-analysis of the rehospitalization rate
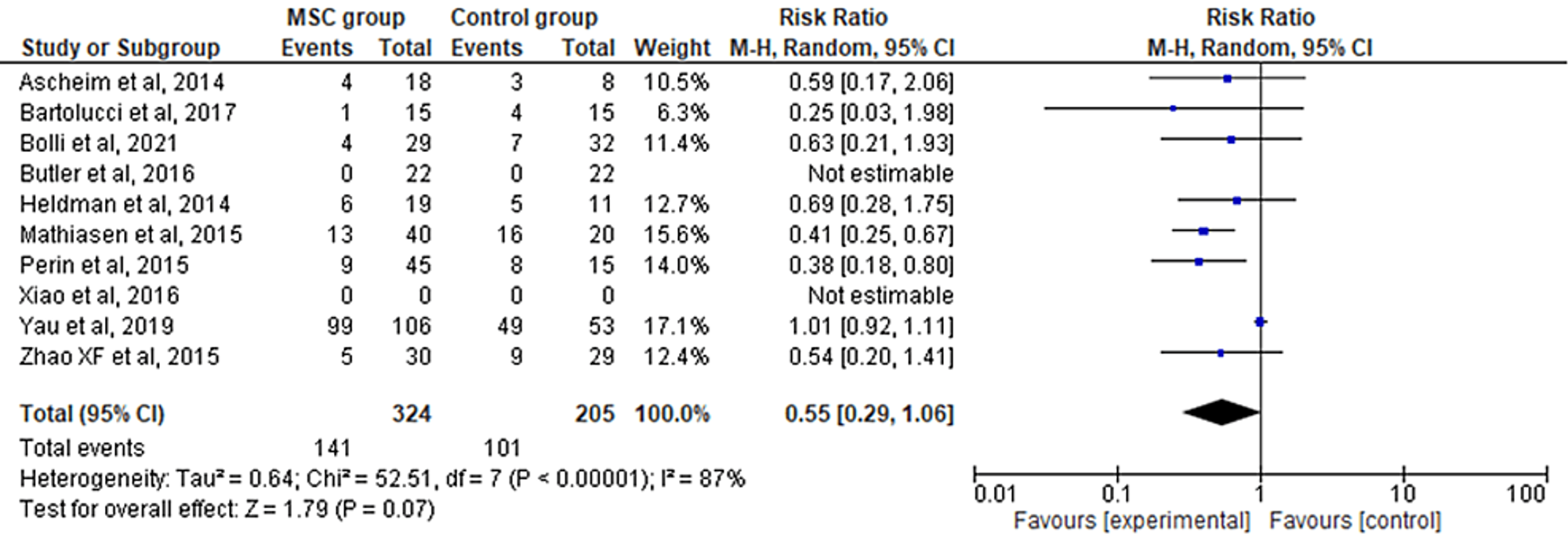
Favors experimental: mesenchymal stem cell (MSC) group.
Myocardial Infarction
The seven included RCTs' pooled estimates indicated that the MSC intervention did not significantly affect myocardial infarction when compared to the control group for HF (RR = 0.41; 95% CI: 0.06-2.76; p = 0.36; I2 = 55%) (Figure 5). To address the heterogeneity in the results, sensitivity analysis was conducted. The results remained consistent, but the heterogeneity lowered considerably (I2 = 0%; heterogeneity p = 0.92) (Appendix D).
Favors experimental: mesenchymal stem cell (MSC) group.
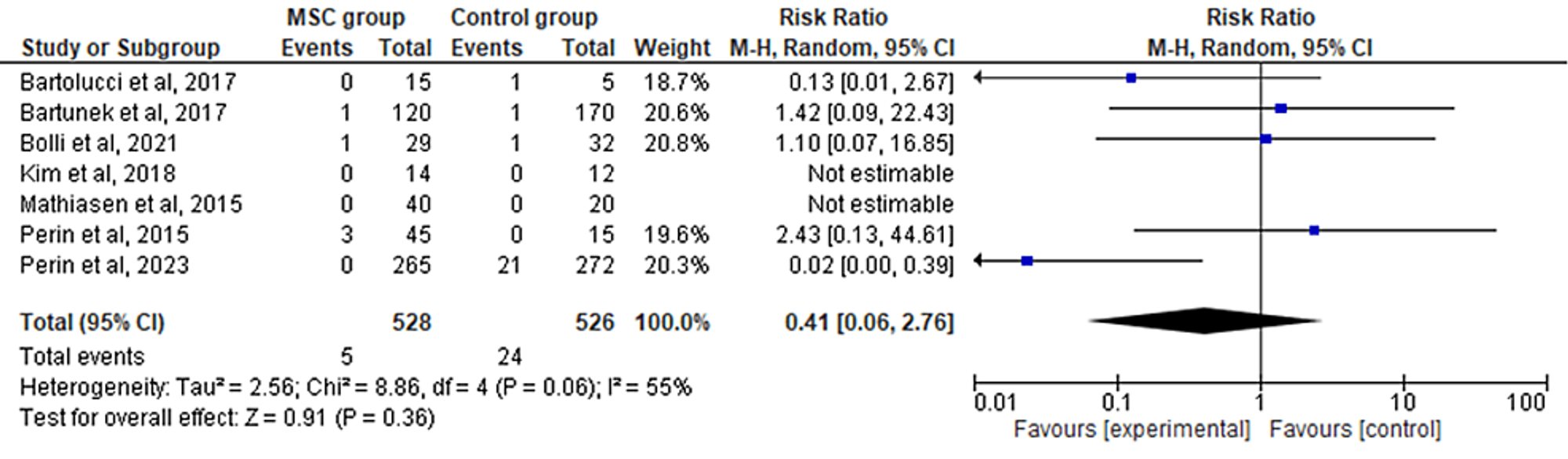
Figure 5: Forest plot for the meta-analysis of myocardial infarction
Recurrence of Heart Failure
The six included RCTs' pooled estimates indicated that the MSC intervention did not significantly affect the recurrence of HF when compared to the control group (RR = 0.74; 95% CI: 0.40-1.37; p = 0.33; I2 = 50%) (Figure 6). To address the heterogeneity in the results, sensitivity analysis was conducted. The results remained consistent, but the heterogeneity lowered considerably (I2 = 0%; heterogeneity p = 0.73) (Appendix E).
Figure 6: Forest plot for the meta-analysis of the recurrence of heart failure
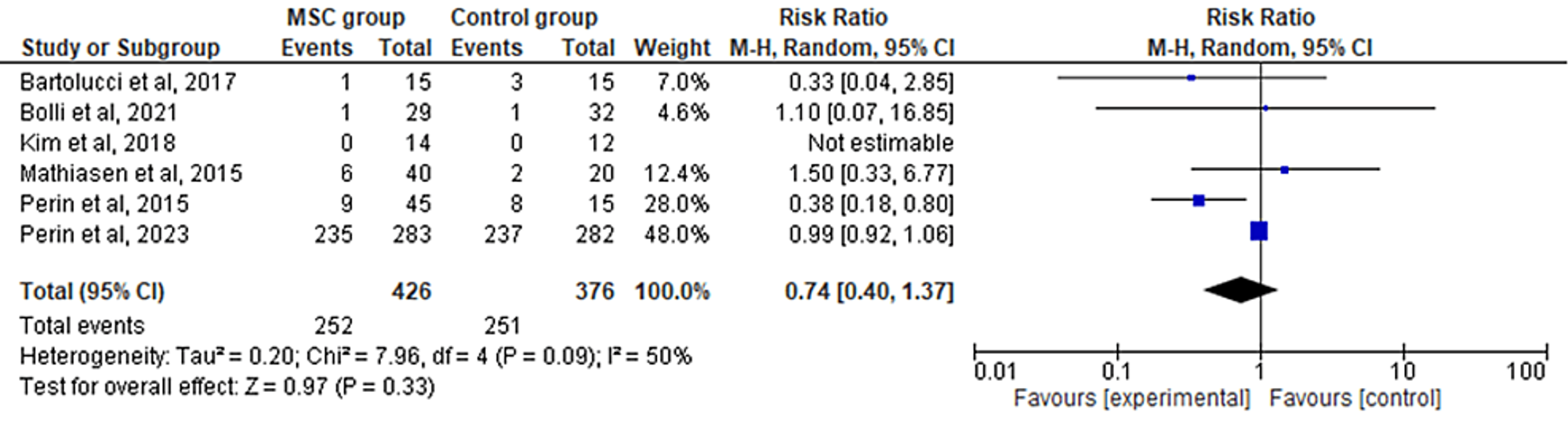
Favors experimental: mesenchymal stem cell (MSC) group.
Total Death
The 12 included RCTs' pooled estimates indicated that the MSC intervention did not significantly affect the total death when compared to the control group (RR = 0.79; 95% CI: 0.52-1.20; p = 0.27) (Figure 7). The heterogeneity between the studies was also low (I2 = 0%; heterogeneity p = 0.84).
Favors experimental: mesenchymal stem cell (MSC) group.
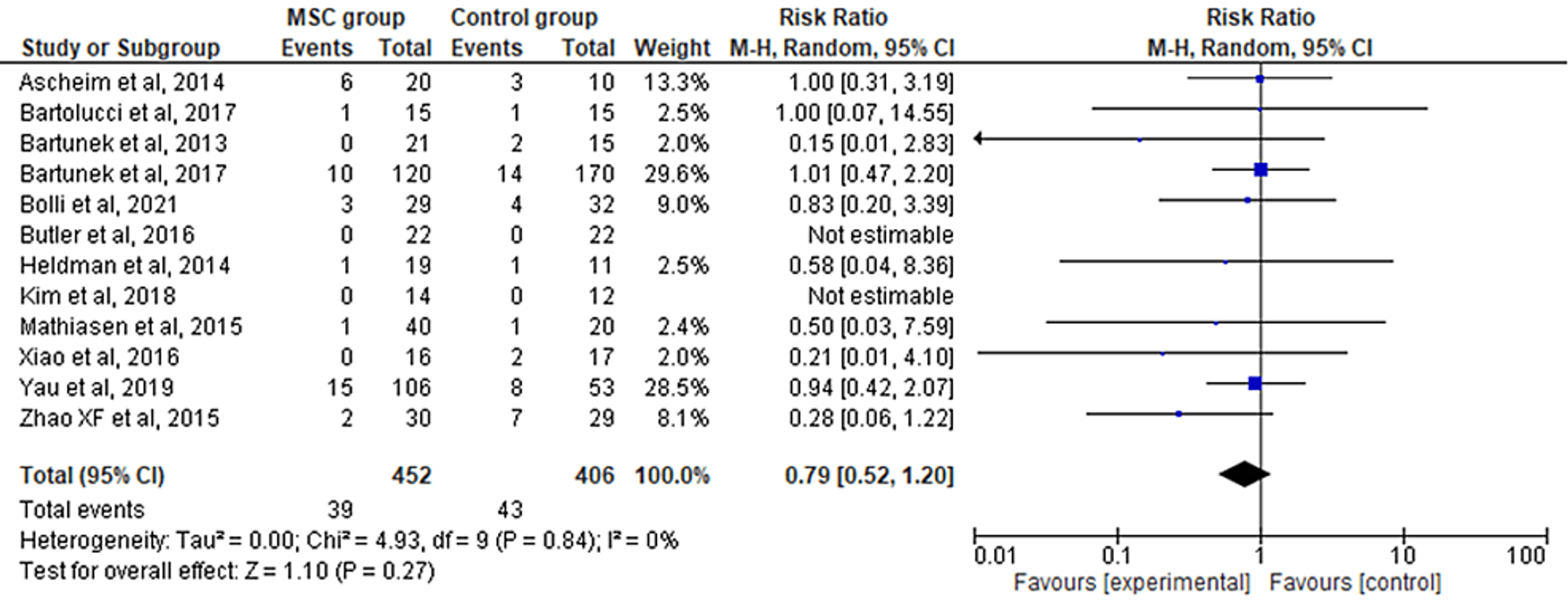
Figure 7: Forest plot for the meta-analysis of total death
Discussion
In our meta-analysis to determine the effect of MSC therapy on outcomes among HF patients, MSC therapy did not affect the outcomes of cardiovascular death, rehospitalization rate, myocardial infarction, recurrence of HF, and total death. However, it was observed that MSC therapy was associated with an increased LVEF as compared to the control group. To address the heterogeneity in the results, sensitivity analysis was conducted. The results remained consistent after sensitivity analysis for the outcomes of myocardial infarction, LVEF, and recurrence of HF. Whereas the results differed for the outcome of rehospitalization rate, favoring the MSC therapy group over the control group.
The clinical effect of MSC therapy for HF patients may be attributed to several processes, including regulation of inflammation, decreased myocardial cell death, myocardial fibrosis, enhanced cell differentiation, and neovascularization. Cell recruitment, migration, and adhesion are only a few of the mechanisms that go into integrating MSCs into tissues. Due to their strong potential for migration and positive reaction to serum in HF patients, umbilical cord MSCs may be able to detect biological cues that are responsible for the therapeutic impact of systemic administration. Our meta-analysis indicates that MSC treatment is linked with considerably improved LVEF and decreased rehospitalization rates when compared to control therapies for HF, but with no significant influence on cardiovascular death [11,22,23].
Previous meta-analyses [24-28] have also been conducted to investigate the association between MSC therapy and adverse or beneficial outcomes in HF patients. Similar to our study, Fan et al. [24] (weighted mean difference (WMD) = 5.25), Fu et al. [25] (mean difference (MD) = 9.64), Jayaraj et al. [26] (MD = 4.58), and Shen et al. [28] (MD = 5.66) also found a significantly improved LVEF on the infusion of MSCs. Moreover, parallel to our findings, Fu et al. [25] found no significant effect of MSCs on cardiovascular death, the occurrence of MI, the recurrence of HF, and total death. However, Lalu et al. [27] found no significant correlation between MSC therapy and LVEF in ischemic HF patients. Furthermore, contrary to our results, Fan et al. [24], Fu et al. [25], and Shen et al. [28] found a significant reduction in rehospitalization rates.
A few existing meta-analyses have also investigated the association between MSCs and manifestations of ischemic heart disease, such as acute myocardial infarction [27,29-31]. It is important to note that ischemic heart disease is a prominent causative agent of HF, and thus it is crucial to review the results of these analyses [32]. While all the aforementioned studies showed improved LVEF in patients suffering from ischemic heart disease, no effect on the risk of readmission and mortality was observed. It can thus be concluded that while MSC therapy significantly improves LVEF and heart function in patients with cardiovascular disease, the overall effect on survival outcomes is insignificant.
Strengths and limitations
Although some previous studies [33,34] have solely evaluated the use of a specific subclass of MSCs, our meta-analysis included studies with all types of MSC therapy, whether it was bone marrow or umbilical cord-derived [11,35]. We also included both types of bone marrow-derived stem cells, autologous and allogeneic. Furthermore, while almost all the existing reviews [24-28] have evaluated LVEF and all-cause mortality, only three [24,25,28] of them have reported data on hospital readmission and one [25] of them has reported data on cardiovascular-specific death. Additionally, we included all studies regardless of the method of delivery of MSCs or type of HF. Whereas Fan et al. [24] included only patients with systolic HF, Lalu et al. [27] included patients with ischemic HF. The presence of only RCTs in our analysis ensures that the risk of bias is minimal [10-19,35-38].
However, due to insufficient data available, we have not evaluated the difference in six-minute walking distance (6MWD) and NYHA class post-therapy, which presents an inevitable limitation of our study. Moreover, no subgroup analysis was done to evaluate the effect of the method of introduction of MSCs in patients or the type of MSC administered. Further research is needed to investigate the effect of specific types of MSC therapy in HF patients.
Conclusions
MSC transplantation results in a significantly improved LVEF. However, due to limited evidence of its effect on survival outcomes and recurrence of HF, more trials should be conducted to investigate the association between this method of treatment and outcomes in HF patients.
References
- Katz AM, Rolett EL: Heart failure: when form fails to follow function. Eur Heart J. 2016, 37:449-54. 10.1093/eurheartj/ehv548
- Heidenreich PA, Albert NM, Allen LA, et al.: Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013, 6:606-19. 10.1161/HHF.0b013e318291329a
- Javaheri S, Barbe F, Campos-Rodriguez F, et al.: Sleep apnea: types, mechanisms, and clinical cardiovascular consequences. J Am Coll Cardiol. 2017, 69:841-58. 10.1016/j.jacc.2016.11.069
- Malik A, Brito D, Vaqar S, Chhabra L: Congestive Heart Failure. StatPearls Publishing, Treasure Island, FL; 2023.
- Chen SL, Fang WW, Qian J, et al.: Improvement of cardiac function after transplantation of autologous bone marrow mesenchymal stem cells in patients with acute myocardial infarction. Chin Med J (Engl). 2004, 117:1443-8.
- Ding DC, Shyu WC, Lin SZ: Mesenchymal stem cells. Cell Transplant. 2011, 20:5-14. 10.3727/096368910X
- Guo Y, Yu Y, Hu S, Chen Y, Shen Z: The therapeutic potential of mesenchymal stem cells for cardiovascular diseases. Cell Death Dis. 2020, 11:349. 10.1038/s41419-020-2542-9
- Cheung MM, Jahan N: Can stem cells improve left ventricular ejection fraction in heart failure? A literature review of skeletal myoblasts and bone marrow-derived cells. Cureus. 2020, 12:e11598. 10.7759/cureus.11598
- Wang Y, Yi H, Song Y: The safety of MSC therapy over the past 15 years: a meta-analysis. Stem Cell Res Ther. 2021, 12:545. 10.1186/s13287-021-02609-x
- Ascheim DD, Gelijns AC, Goldstein D, et al.: Mesenchymal precursor cells as adjunctive therapy in recipients of contemporary left ventricular assist devices. Circulation. 2014, 129:2287-96. 10.1161/CIRCULATIONAHA.113.007412
- Bartolucci J, Verdugo FJ, González PL, et al.: Safety and efficacy of the intravenous infusion of umbilical cord mesenchymal stem cells in patients with heart failure: a phase 1/2 randomized controlled trial (RIMECARD trial [Randomized Clinical Trial of Intravenous Infusion Umbilical Cord Mesenchymal Stem Cells on Cardiopathy]). Circ Res. 2017, 121:1192-204. 10.1161/CIRCRESAHA.117.310712
- Bartunek J, Behfar A, Dolatabadi D, et al.: Cardiopoietic stem cell therapy in heart failure: the C-CURE (cardiopoietic stem cell therapy in heart failure) multicenter randomized trial with lineage-specified biologics. J Am Coll Cardiol. 2013, 61:2329-38. 10.1016/j.jacc.2013.02.071
- Bolli R, Mitrani RD, Hare JM, et al.: A phase II study of autologous mesenchymal stromal cells and c-kit positive cardiac cells, alone or in combination, in patients with ischaemic heart failure: the CCTRN CONCERT-HF trial. Eur J Heart Fail. 2021, 23:661-74. 10.1002/ejhf.2178
- Heldman AW, DiFede DL, Fishman JE, et al.: Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: the TAC-HFT randomized trial. JAMA. 2014, 311:62-73. 10.1001/jama.2013.282909
- Mathiasen AB, Qayyum AA, Jørgensen E, et al.: Bone marrow-derived mesenchymal stromal cell treatment in patients with severe ischaemic heart failure: a randomized placebo-controlled trial (MSC-HF trial). Eur Heart J. 2015, 36:1744-53. 10.1093/eurheartj/ehv136
- Perin EC, Borow KM, Henry TD, et al.: Randomized trial of targeted transendocardial mesenchymal precursor cell therapy in patients with heart failure. J Am Coll Cardiol. 2023, 81:849-63. 10.1016/j.jacc.2022.11.061
- Perin EC, Borow KM, Silva GV, et al.: A phase II dose-escalation study of allogeneic mesenchymal precursor cells in patients with ischemic or nonischemic heart failure. Circ Res. 2015, 117:576-84. 10.1161/CIRCRESAHA.115.306332
- Bartunek J, Terzic A, Davison BA, et al.: Cardiopoietic cell therapy for advanced ischaemic heart failure: results at 39 weeks of the prospective, randomized, double blind, sham-controlled CHART-1 clinical trial. Eur Heart J. 2017, 38:648-60. 10.1093/eurheartj/ehw543
- Yau TM, Pagani FD, Mancini DM, et al.: Intramyocardial injection of mesenchymal precursor cells and successful temporary weaning from left ventricular assist device support in patients with advanced heart failure: a randomized clinical trial. JAMA. 2019, 321:1176-86. 10.1001/jama.2019.2341
- Selçuk AA: A guide for systematic reviews: PRISMA. Turk Arch Otorhinolaryngol. 2019, 57:57-8. 10.5152/tao.2019.4058
- Minozzi S, Cinquini M, Gianola S, Gonzalez-Lorenzo M, Banzi R: The revised Cochrane risk of bias tool for randomized trials (RoB 2) showed low interrater reliability and challenges in its application. J Clin Epidemiol. 2020, 126:37-44. 10.1016/j.jclinepi.2020.06.015
- Gong X, Wang P, Wu Q, Wang S, Yu L, Wang G: Human umbilical cord blood derived mesenchymal stem cells improve cardiac function in cTnT(R141W) transgenic mouse of dilated cardiomyopathy. Eur J Cell Biol. 2016, 95:57-67. 10.1016/j.ejcb.2015.11.003
- Huang J, Zhang Z, Guo J, et al.: Genetic modification of mesenchymal stem cells overexpressing CCR1 increases cell viability, migration, engraftment, and capillary density in the injured myocardium. Circ Res. 2010, 106:1753-62. 10.1161/CIRCRESAHA.109.196030
- Fan M, Huang Y, Chen Z, et al.: Efficacy of mesenchymal stem cell therapy in systolic heart failure: a systematic review and meta-analysis. Stem Cell Res Ther. 2019, 10:150. 10.1186/s13287-019-1258-1
- Fu H, Chen Q: Mesenchymal stem cell therapy for heart failure: a meta-analysis. Herz. 2020, 45:557-63. 10.1007/s00059-018-4762-7
- Jayaraj JS, Janapala RN, Qaseem A, et al.: Efficacy and safety of stem cell therapy in advanced heart failure patients: a systematic review with a meta-analysis of recent trials between 2017 and 2019. Cureus. 2019, 11:e5585. 10.7759/cureus.5585
- Lalu MM, Mazzarello S, Zlepnig J, et al.: Safety and efficacy of adult stem cell therapy for acute myocardial infarction and ischemic heart failure (SafeCell Heart): a systematic review and meta-analysis. Stem Cells Transl Med. 2018, 7:857-66. 10.1002/sctm.18-0120
- Shen T, Xia L, Dong W, et al.: A systematic review and meta-analysis: safety and efficacy of mesenchymal stem cells therapy for heart failure. Curr Stem Cell Res Ther. 2021, 16:354-65. 10.2174/1574888X15999200820171432
- Attar A, Bahmanzadegan Jahromi F, Kavousi S, Monabati A, Kazemi A: Mesenchymal stem cell transplantation after acute myocardial infarction: a meta-analysis of clinical trials. Stem Cell Res Ther. 2021, 12:600. 10.1186/s13287-021-02667-1
- Jeong H, Yim HW, Park HJ, Cho Y, Hong H, Kim NJ, Oh IH: Mesenchymal stem cell therapy for ischemic heart disease: systematic review and meta-analysis. Int J Stem Cells. 2018, 11:1-12. 10.15283/ijsc17061
- Yu J, Zhang RF, Mao YL, Zhang H: Efficacy and safety of mesenchymal stem cell therapy in patients with acute myocardial infarction: a systematic review and meta-analysis of randomized controlled trials. Curr Stem Cell Res Ther. 2022, 17:793-807. 10.2174/1574888X16666210816111031
- Severino P, D'Amato A, Pucci M, et al.: Ischemic heart disease and heart failure: role of coronary ion channels. Int J Mol Sci. 2020, 21:3167. 10.3390/ijms21093167
- Kalou Y, Al-Khani AM, Haider KH: Bone marrow mesenchymal stem cells for heart failure treatment: a systematic review and meta-analysis. Heart Lung Circ. 2023, 32:870-80. 10.1016/j.hlc.2023.01.012
- Sato Y, Kuragaichi T, Saga S, et al.: Safety of intravenous autologous bone marrow-derived mesenchymal cell transplantation in 5 patients with reduced left ventricular ejection fraction. Circ Rep. 2021, 3:550-4. 10.1253/circrep.CR-21-0091
- Zhao XF, Xu Y, Zhu ZY, Gao CY, Shi YN: Clinical observation of umbilical cord mesenchymal stem cell treatment of severe systolic heart failure. Genet Mol Res. 2015, 14:3010-7. 10.4238/2015.April.10.11
- Butler J, Epstein SE, Greene SJ, et al.: Intravenous allogeneic mesenchymal stem cells for nonischemic cardiomyopathy: safety and efficacy results of a phase II-A randomized trial. Circ Res. 2017, 120:332-40. 10.1161/CIRCRESAHA.116.309717
- Kim SH, Cho JH, Lee YH, et al.: Improvement in left ventricular function with intracoronary mesenchymal stem cell therapy in a patient with anterior wall ST-segment elevation myocardial infarction. Cardiovasc Drugs Ther. 2018, 32:329-38. 10.1007/s10557-018-6804-z
- Xiao W, Guo S, Gao C, et al.: A randomized comparative study on the efficacy of intracoronary infusion of autologous bone marrow mononuclear cells and mesenchymal stem cells in patients with dilated cardiomyopathy. Int Heart J. 2017, 58:238-44. 10.1536/ihj.16-328
Appendices
Appendix A
Figure 8: Quality assessment of all the included studies using the risk of bias tool 2.0
Appendix B
Figure 9: Sensitivity analysis on the outcome of left ventricular ejection fraction
MSC: mesenchymal stem cell.
Appendix C
Figure 10: Sensitivity analysis on the outcome of left ventricular ejection fraction
MSC: mesenchymal stem cell.
Appendix D
Figure 11: Sensitivity analysis on the outcome of myocardial infarction
MSC: mesenchymal stem cell.
Appendix E
Figure 12: Sensitivity analysis on the outcome of recurrence of heart failure
MSC: mesenchymal stem cell.
Related Topics
Further Reading
Aortic Dissection Associated With Seizures
Self-Perception of Aging and Hypertension in a Cohort of Sexual Minority
A Case of Asymptomatic Massive Inguinoscrotal Bladder in Acute Renal Failure